Jinyan Tao
Can you picture such a scenario? You are having a morning tea, a sudden feeling of stomachache hits you, so you open a medical advising App on your personal mobile device which records your body temperature, movement and stores your genetic profile and previous medical records. Then you type in: having a tea, feeling unwell, maybe a stomachache. Within a few seconds, the App gives you feedback instructing you to take certain medications, just with one click to accept the advice, your medications will be delivered to your address on the same day.
The description above is not a fantasy, it is an achievable future with the fundamentals and integration of medical care (prevention, detection, diagnosis and treatment), big data and social economics, which can offer tailored medical solutions to individuals based on personal characteristics i.e. molecular and genetic profiles. A concept of “so-called” personalized medicine, a promising future healthcare solution.(Hizel et al., 2017)
On the contrary of traditional healthcare strategy: providing a general therapy solution applies to the whole population (one-for-all solution), personalized medicine is the tailoring of medical treatment to the individual characteristics of each patient. The theoretical scientific basis for personalized therapy is the varying individual response and safety profile to a certain treatment, which results from the unique molecular and genetic characteristics in each patient. Tailoring a personalized therapy can optimize individual treatment response and safety margin, decrease adverse effects and lower healthcare expense. With growing elucidation on disease etiology and progression on a molecular level, as well as technology breakthroughs in next-generation sequencing, digital health and artificial intelligence, personalized medicine shows great potential in not only providing rational and precise treatment for patients but the strategy in prediction, prophylaxis of diseases. (Mathur and Sutton, 2017, PersonalizedMedicineCoalition)
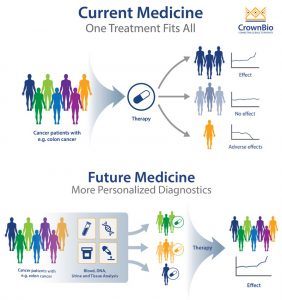
(Barbeau, 2018)
Foundation and progress of precision medicine
Human genetic mapping and next-generation sequencing
Research on DNA function and molecular mechanism clarification is the foundation of personalized medicine.
Great accomplishments of the human genome project in 2003 enabled deciphering of the enigmas in human DNA, a total 22300 protein-coding genes, how they govern and control the growth and function of human bodies. Thereafter, the boost of genomics studies never ceases to unravel association between genotypes and disease occurrence. Meanwhile, the advances in high through-put next-generation sequencing (NGS) technology extensively lowered cost of whole genome sequencing to 100 dollars per sample. With the robustness and acceleration of NGS, personal genetic profile can be rapidly constructed and easily analysed to offer insights on early disease diagnosis and progression.(Dhawan, 2017)
Nowadays, successful early cancer drug discovery and development highly relies on cancer genomics. As broadly implemented in clinical cancer research and diagnosis as it is, clinical gene sequencing targeted for specific gene variants among cancer patients provides evidence for altered response to certain treatment regimens due to genetic profile variance. There are a few antibody treatments to several cancer types with proven efficacy and safety profile among targeted population on the market. Herceptin, as one of the most famous and effective anti-breast cancer protein products, it specifically targets the HER-2 positive breast cancer patients with less adverse effects and improved efficacy compared with traditional chemotherapy.(Reynolds et al., 2014)
The big data and artificial intelligence
Having entered the 21st century, the thriving of data science and artificial intelligence are constantly transforming a number of traditional businesses and reforming the way we look at data. In healthcare, tons of data are generated on a daily basis without being fully explored and exploited. Genomics data aforementioned are comparatively well maintained and investigated throughout years of formation of quality standards. In the biomedical informatic research, initiatives like the International Hap Map Project, The Cancer Genome Atlas (TCGA) and others are proven success that -omics datasets stored in well-managed databank can serve as tool for scientific development and health care improvement. (Hernández-Lemus et al., 2017)
Despite data stored for research purpose, recently emerging analysis on electronic health records (EHRs) and unveiled huge exploitation capacity based on personal data. Electronic health records cover a wide range of information includes but not limited to biochemical tests and laboratory values, text-based documents, as well as historical accounts of medical interventions, therapies, and pharmaceutical deliveries. With integration and proper organization and adequate combination with individual genetic profile, a comprehensive personal health profile can be constructed. Moreover, electronic data can be easily accessed and analyzed by data scientist or automatedly acquired by doctors. There are monitoring systems implemented in hospitals in Germany, based on previous medical record of patients and in combination with computational algorithms and software platforms, systems can generate medical alerts to help prevent onset of diseases e.g. epilepsy or prescriptions of risky drug combinations. (Baumgartner et al., 2018, Pirnejad et al., 2019)
Meanwhile, computational modelling with support of statistical analysis and computing power significantly elevates the data handling capacity, the mining of seemingly unorganized massive datasets using statistics or machine learning via hypothesis testing makes the hidden information more perceivable and presentable, it can provide predictive ability on non-tested questions. However, one still needs sufficient and scientific training for the proper formation of questions by observing and analyzing datasets, then to apply justifiable computational and analytical tools in answering questions raised.
The infamously long-lasting and costly clinical trials, I can think of, can take benefits from the implementation of data mining and AIs. By predicting beneficiary population based on genetic and molecular profiles, subjects can be narrowed down and recruited, which would lower clinical cost and generate accumulatively favored clinical outcomes. Cost-effectiveness-wise, shortened trials along with improved success rate, more effective products can be brought to the market at a lower price.
Besides AI and data mining, digital health plays a crucial part in personalized medicine. The idea of recording/monitoring personal health parameters with a portable mobile device is bringing many AI-driven diagnostic applications on the market. Some of them provide insights on early diagnosis for clinically undetectable neurodegenerative diseases such as Parkinson disease by recording and analyzing personal motions based on AI and motional data. Booming studies on Image recognition also providing inspiring insights on AI- diagnosis. With combinations of multiple disciplines, personalized medicine is showing us an alternative of future healthcare.(Uddin et al., 2019)
Challenges
What is coming after emerging opportunities, are always challenge. Although many innovative technologies contribute to the development of personalized medicine, there are certainly some problems to be tackled, be they either technical or ethical.
What can be most widely discussed would be personal data protection and privacy. Many would raise questions concerning the legitimacy of data collection, maintenance and protection. Especially under the policy of The General Data Protection Regulation (GDPR) in Europe, the collection and certain usage of personal health data should first of all obtain the consents from subjects. For clinical trial and mobile health data, robust data protection safeguards should apply, and data usage should, without further authorization, be restricted to conditions and terms that are included in subject consents. However, the protection itself would require additional efforts and investments on both technical and legislation perspectives with the emergence of innovative tools.(EuropeanDataProtectionSupervisor, Daniel E. Hall MD MDiv, March 20, 2012)
Besides, data management and quality control play an essential role for the subsequent analysis and mining. Today, many data are being generated and maintained around the globe in ununiformed standards, bringing a major hindrance for the integration of all data available. Although there are some data initiatives proving data management success as mentioned before, it is necessary to call for a much wider range of uniform data integration and standardization.
On a more technical term, the computational challenges are major for implementation of fancy idea in precision healthcare. Maintaining computing capacity and storage for massive amounts of data is quite costly. Performing analysis on large dataset can be quite computationally expensive, which is much less affordable for individual analyst than for large institutions equipped with high performance computing power. Moreover, biomedical data exploration highly relies on the development of data processing software platforms and powerful algorithms. The advances in computational technologies created a new “ecological” environment for data analysis, certainly are making it more tangible, accessible and efficient, however, is there a ceiling or up limit where computational technologies can not break? The answer is quite certain: human intelligence.(Hernández-Lemus et al., 2017)
Even with many previous emphases on computational technology breakthroughs, in terms of healthcare issues, human intelligence can never be replaced. Personalized medicine, after all, is a strategy to offer tailored therapy, to maximize therapeutic cost-effectiveness. The serving and served subjects are nevertheless human beings. Computational technology, in the end, will always serve as a tool to suggest plausible therapeutic options derived from statistical analysis of gathered information and evidences. However powerful and predictive the tool might be, the rational final decision, I believe, will anyhow be made by human intelligence.
Bibliography
BAUMGARTNER, C., KOREN, J. P. & ROTHMAYER, M. 2018. Automatic Computer-Based Detection of Epileptic Seizures. Front Neurol, 9, 639.
DANIEL E. HALL MD MDIV, A. V. P. M. M., AARON S. FINK MD March 20, 2012. Informed consent for clinical treatment. CMAJ, 8.
DHAWAN, D. 2017. Clinical Next-Generation Sequencing. Progress and Challenges in Precision Medicine.
EUROPEANDATAPROTECTIONSUPERVISOR. https://edps.europa.eu/data-protection/our-work/subjects/health_en. [Accessed].
HERNÁNDEZ-LEMUS, E., ESPINAL-ENRÍQUEZ, J. & GARCÍA-HERRERA, R. 2017. Handling Big Data in Precision Medicine. Progress and Challenges in Precision Medicine.
HIZEL, C., TREMBLAY, J., BARTLETT, G. & HAMET, P. 2017. Introduction. Progress and Challenges in Precision Medicine.
MATHUR, S. & SUTTON, J. 2017. Personalized medicine could transform healthcare. Biomed Rep, 7, 3-5.
PERSONALIZEDMEDICINECOALITION. What is personalized medicine? [Online]. http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/pmc_age_of_pmc_factsheet.pdf. [Accessed].
PIRNEJAD, H., AMIRI, P., NIAZKHANI, Z., SHIVA, A., MAKHDOOMI, K., ABKHIZ, S., VAN DER SIJS, H. & BAL, R. 2019. Preventing potential drug-drug interactions through alerting decision support systems: A clinical context based methodology. Int J Med Inform, 127, 18-26.
REYNOLDS, K., SARANGI, S., BARDIA, A. & DIZON, D. S. 2014. Precision medicine and personalized breast cancer: combination pertuzumab therapy. Pharmgenomics Pers Med, 7, 95-105.
UDDIN, M., WANG, Y. & WOODBURY-SMITH, M. 2019. Artificial intelligence for precision medicine in neurodevelopmental disorders. NPJ Digit Med, 2, 112.
Barbeau, J., 2018. CrownBio. [Online]
Available at: https://blog.crownbio.com/pdx-personalized-medicine#_
Media Attributions
- current-medicine-future-medicine