Muriel Cottagnoud
Malaria, a well-known illness that might have fallen into oblivion in Europe but is still omnipresent in African regions. Mosquitos trans the illness-causing parasites to humans and cause hundreds of thousands of deaths per year. Although the fight against malaria has been a success story due to prevention tools, there is still a great potential to lower the mortality rate. Biotechnology could be a new game changer in this fight, permanently changing the mosquitos’ genome.
Malaria in General
Malaria is an acute febrile illness caused by the plasmodium parasites. P. falciparum, P. vivax, P. ovale, P. malariae P. knowlesi are the five parasites causing the illness in humans. Only the female Anopheles gambiae (A. gambiae) can pass the parasites on to humans through bites, making the mosquitos the malaria vector. Infected individuals will at first suffer from fever, headaches, muscle pain and might even suffer from cerebral malaria. This is when the parasite attacks the brain, causing seizures (BAG, 2021).
 In 2020, 627,000 deaths were caused by malaria and 241 million cases were registered by the WHO. 95% of the cases and 96% of the deaths due to malaria were registered in African countries, making Africa the most affected region in the world (WHO, 2022). According to the WHO (2022) there are four countries that accounted for more than half of all malaria deaths in 2020: Nigeria (31.9%), the Democratic Republic of the Congo (13.2%), United Republic of Tanzania (4.1%) and Mozambique (3.8%). Several malaria preventions tools – vector control, preventive chemotherapy and vaccine – have been in use for over the two decades and led to a mortality rate drop of 80% in Tanzania from 2000 to 2015 (Gates, 2016). Vector control is the application of insecticide-treated nets and indoor spraying. Preventive chemotherapy is the use of medicine, preventing malaria infection and lowering its symptoms. The third tool is a malaria vaccine for children that is dosed for children from an age of five months (WHO, 2022). With new and better technologies and understanding of the environment, a new and fourth tool has come up that could prevent malaria: genome editing. The aim of genome editing is to change the genotype of the malaria vector.
In 2020, 627,000 deaths were caused by malaria and 241 million cases were registered by the WHO. 95% of the cases and 96% of the deaths due to malaria were registered in African countries, making Africa the most affected region in the world (WHO, 2022). According to the WHO (2022) there are four countries that accounted for more than half of all malaria deaths in 2020: Nigeria (31.9%), the Democratic Republic of the Congo (13.2%), United Republic of Tanzania (4.1%) and Mozambique (3.8%). Several malaria preventions tools – vector control, preventive chemotherapy and vaccine – have been in use for over the two decades and led to a mortality rate drop of 80% in Tanzania from 2000 to 2015 (Gates, 2016). Vector control is the application of insecticide-treated nets and indoor spraying. Preventive chemotherapy is the use of medicine, preventing malaria infection and lowering its symptoms. The third tool is a malaria vaccine for children that is dosed for children from an age of five months (WHO, 2022). With new and better technologies and understanding of the environment, a new and fourth tool has come up that could prevent malaria: genome editing. The aim of genome editing is to change the genotype of the malaria vector.
Genome Editing on the Malaria Vector
There are two strategies to alter a malaria vector population’s genotype. The first strategy is to suppress female fertility, the second is to insert a sex distorted Y chromosome. The second strategy has been proven to be ineffective, since during meiosis the sex chromosomes are shut down, leading to an unsuccessful expression of the altered Y chromosome (K
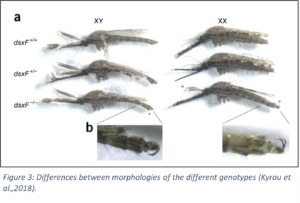
 yrou et al., 2018). However, the first method, using CRISPR-Cas9 has been proven successful. CRISPR-Cas9 method can be applied to modify a specific DNA region in the female mosquito A. gambiae. According to the study of Kyrou et al. (2018) a disrupted gene region of the intron 4-exon 5 boundary will result in a malfunctioning AgdsxF gene. AgdsxF is a doublesex (recessive) gene that encodes for two transcripts dsx-female (AgdsxF) and dsx-male (AgdsxM) (Figure 2) and hence will determine the two sexes of a A.gambiae mosquito. For the experiment Cas9a, single-strangled RNA and eGFP were injected into the gametes of A. gambiae. The RNA was created to specifically recognize the overlapping intron 4-exon 5 boundary. Additionally, the RNA was created to affect only the AgdsxF, leaving AgdsxM (sex determination for males) intact. The presence of the GFP altered the genetic pattern of the exon 5, resulting in a disruption of the exon 5. The modified embryos were then intercrossed with each other and generated homozygous and heterozygous mutants. Among the offspring, individuals with homozygous genotype of dsxF-/- not only showed an affected reproductive system but also showed anomalies in sexual morphology. Females with dsxF-/- were characterized by the absence of fully developed ovaries that were replaced with male glandes. Further, in 20% of the cases females even showed an archetype of organs that look like unstructured testes.As mentioned above dsxF-/- females also revealed anomalies in sexual morphology (Figure 3, part A). These individuals showed male specific traits such as the plumose antenna (Figure 3, part B). These alterations result in the inability to feed on blood and produce eggs. Not being able to take blood meals means that their bites to humans will become harmless. The effects of the inability to produce eggs eventually led to the collapse of the A. gambiae population in the 8th generation (Kyrou et al., 2018).
yrou et al., 2018). However, the first method, using CRISPR-Cas9 has been proven successful. CRISPR-Cas9 method can be applied to modify a specific DNA region in the female mosquito A. gambiae. According to the study of Kyrou et al. (2018) a disrupted gene region of the intron 4-exon 5 boundary will result in a malfunctioning AgdsxF gene. AgdsxF is a doublesex (recessive) gene that encodes for two transcripts dsx-female (AgdsxF) and dsx-male (AgdsxM) (Figure 2) and hence will determine the two sexes of a A.gambiae mosquito. For the experiment Cas9a, single-strangled RNA and eGFP were injected into the gametes of A. gambiae. The RNA was created to specifically recognize the overlapping intron 4-exon 5 boundary. Additionally, the RNA was created to affect only the AgdsxF, leaving AgdsxM (sex determination for males) intact. The presence of the GFP altered the genetic pattern of the exon 5, resulting in a disruption of the exon 5. The modified embryos were then intercrossed with each other and generated homozygous and heterozygous mutants. Among the offspring, individuals with homozygous genotype of dsxF-/- not only showed an affected reproductive system but also showed anomalies in sexual morphology. Females with dsxF-/- were characterized by the absence of fully developed ovaries that were replaced with male glandes. Further, in 20% of the cases females even showed an archetype of organs that look like unstructured testes.As mentioned above dsxF-/- females also revealed anomalies in sexual morphology (Figure 3, part A). These individuals showed male specific traits such as the plumose antenna (Figure 3, part B). These alterations result in the inability to feed on blood and produce eggs. Not being able to take blood meals means that their bites to humans will become harmless. The effects of the inability to produce eggs eventually led to the collapse of the A. gambiae population in the 8th generation (Kyrou et al., 2018).
Benefits and Risks of Biotechnology
The use of innovative biotechnologies always goes along with benefits and risks, not to mention ethical considerations, since it is an invasion in nature and its ecosystems.
First of all, the CRISPR-Cas9 is a simple and cheap method that can permanently change a specific DNA region. This can result in the eradication of infectious diseases. More specifically, the CRISPR-Cas9’s usage to change the mosquito’s genome can have a crucial impact on human health; its immediate benefit is to fight malaria and save lives (Neves & Druml, 2017).Yet, it is to be questioned whether science can guarantee an errorless exposure for humans to the modified mosquitos. According to Kyrou et al. (2018), the study cannot support the development of a collapse of the malaria vector population responsible for malaria transmission. Further, the study cannot show a resistance-proof for the dsxF-/- gene, meaning that due to nuclease mutations the genotype of the doublesex gene might change and then be positively selected (Kyrou et al., 2018).
A risk of biotechnologies is according to Neves & Druml (2017) the impact on the ecosystems and biodiversity. This debate marks a shift from scientific to ethical consideration. Altering a mosquito’s genome will result in the reduction of biodiversity and may cause damage to the ecosystems. These scenarios are not fully understood yet and more research needs to be done in this field. Another side effect of genome editing is the invasion in nature, causing the conflict with the idea of nature and how nature should be independent to human purposes (Neves & Druml, 2017).
Once the usage of CRISPR-Cas9 is applied to mosquitos, it will inevitably be used in other insects and species, which could further unpredictable consequences. It is to be questioned which institutions will have the rights to decide its applications and to which species. Where are the boundaries to be drawn in order to prevent too severe an invasion of nature?
Finally, the decision of innovative biotechnologies use should include African communities, since they are affected the most by malaria. Often, they lack capacity for an informed debate and free decision-making (Neves & Druml, 2017). Therefore, how can it be assured that the affected communities have access to a non-biased information resource before being exposed to gene-modified mosquitos?
In conclusion, genome editing has a big potential for winning the fight against malaria and therefore can save many lives. Its cheap and sustainable characteristics makes it very lucrative. Nevertheless, it still lacks scientific evidence that when exposed to nature and humans, it will not have any side effects that could cause even greater harm.
Ethical considerations such as how far human needs are allowed to invade nature need to be considered first before using the technique in every day’s life.
References
BAG. (2021). Malaria. Retrieved from https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/malaria.html
Gates, B. (2016). Mapping the end of malaria. Retrieved from https://www.gatesnotes.com/Health/Mapping-the-End-of-Malaria
Kyrou, K., Hammond, A. M., Galizi, R., Kranjc, N., Burt, A., Beaghton, A. K., … Crisanti, A. (2018). A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nature Biotechnology, 36(11), 1062–1071. Retrieved from https://doi.org/10.1038/nbt.4245
Neves, M. P., & Druml, C. (2017). Ethical implications of fighting malaria with CRISPR/Cas9. BMJ Global Health, 2(3), 9–11. Retrieved from https://doi.org/10.1136/bmjgh-2017-000396
WHO. (2022). Malaria. Retrieved from https://www.who.int/news-room/fact-sheets/detail/malaria